HUAREN MEDICAL TECHNOLOGY
R & D SERVICES
CDMO Services for Cell Therapy Products
CDMO Services for Cell Therapy Products

CDMO Services for Cell Therapy Products
Facilitate the R&D and industrialized production of cell therapy products and accelerate the process of bringing such products to market
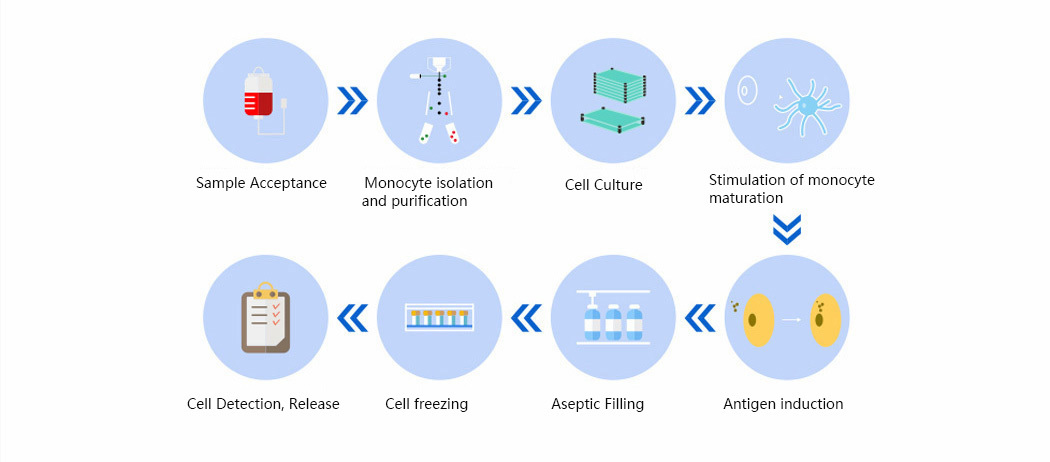
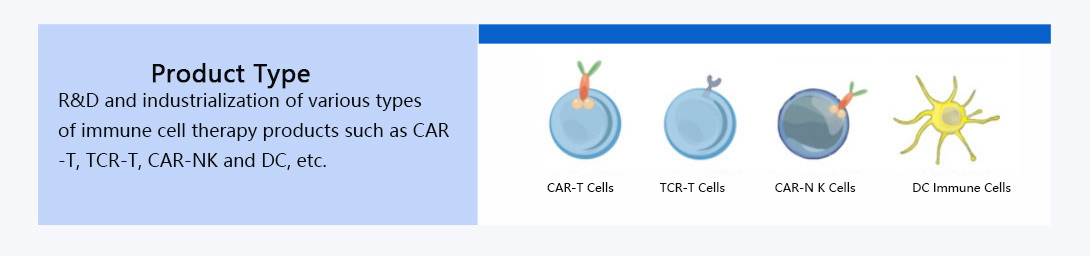
We provide CTDMO services integrating development, manufacturing and testing for autologous and allogeneic cell therapies. The platform process is applicable to cells from various sources (e.g. peripheral blood, bone marrow, adipose, etc.), and we engage in process development and scale-up manufacturing of CAR-T, NK, DC and other immune cellular therapies, analytical method development and quality research, and production and release of clinical samples.
• Scope of Services
• Immune cell therapies development route planning and design based on QbD concepts
• Industrialization-oriented immune cell therapies process development and scale-up
• Immune cell therapies quality control and strategy consulting
• Analytical method development and quality study compliant with filing requirements both at home and abroad
• Pharmacological studies and IND filing preparation for immune cell therapies
• Clinical trial sample production and Large-scale contract manufacturing of cellular therapies for clinical trials

• Manufacturing Platform
• The CTDMO platform for cell therapy products is based on the QbD concept. Our laboratory completes the filing of a Level II Biosafety Laboratory, and is equipped with a separate HAVC and full-exhaust system. We adopt a closed flexible manufacturing process, which helps reduce the cost of production and effectively avoid contamination and cross-contamination.
• 2D and 3D cell culture using flasks, stirrers and wave bioreactors are available for flexible cell expansion to meet the different scale requirements of customers.
• We are proud to have a well-established quality management system with complete production traceability and compliance. And we provide customers with faster, more convenient, safe and effective immune cell therapy products.

• Manufacturing Process
CAR-T manufacturing process

Cell Banking Process