HUAREN MEDICAL TECHNOLOGY
STEM CELLS
Iron death and the feud over mesenchymal stem cell therapy


▲Copyrighted image, please do not reproduce
01 INTRODUCTION
Iron death? What was your first thought? Did you think that iron also dies? If you think so, you are wrong, Iron death is a new type of regulated cell death, this unique mode of cell death is driven by iron-dependent phospholipid peroxidation, regulated by a variety of cellular metabolic events, and is involved in a number of signaling pathways related to disease development. Since the emergence of the term "iron death" in 2012, the research on iron death has increased exponentially in recent years, and has been recognized as a research hotspot of the National Natural Science Foundation of China for consecutive years. Today, I would like to talk about what iron death is, what diseases iron death plays an important role, and whether mesenchymal stem cells, which have made a big splash in the field of therapeutics, also have a significant role in disease damage induced by iron death. The mesenchymal stem cells have an unexpected effect on iron death-induced disease damage.
02 What is iron death?
Iron death is defined as iron-dependent, regulated death caused by rupture of the plasma membrane due to unrestricted lipid peroxidation [1]. This process relies on a combination of metabolites reactive oxygen species (ROS), phospholipids of polyunsaturated fatty acid chains (PUFA-PL), and the transition metal iron [2]. Iron death can be caused by extrinsic or intrinsic pathways; the extrinsic pathway is initiated by inhibition of cell membrane transport proteins such as cystine/glutamate transport proteins or activation of iron transfer proteins, serum transferrin and lactotransferrin; and the intrinsic pathway is activated by the blockade of intracellular antioxidant enzymes such as glutathione peroxidase GPX4 [3]. In addition to small molecule compounds and drugs, intracellular and intercellular signaling events and environmental stresses (e.g., high temperature, low temperature, hypoxia, and radiation) can induce iron death, and aberrant regulation of this process has been linked to protein degradation pathways (e.g., autophagy and the ubiquitin-proteasome system), which are closely associated with a variety of pathological conditions such as cardiovascular disease, inflammation, cancer, and neurodegenerative disease (Figure 1).

▲Fig. 1 Overview of iron death [2].
03 The role of iron death in cardiovascular disease
The maintenance of iron homeostasis is critical for normal cardiac function, and there is growing evidence that iron death is a common feature of many cardiovascular disease subtypes, such as adriamycin-induced cardiomyopathy, myocardial ischemia-reperfusion injury, myocardial infarction, and heart failure [4]. It has been shown that iron death during cardiac ischemia-reperfusion promotes the release of endogenous substances through Toll-like receptor 4-dependent signaling pathways, causing adhesion of neutrophils to coronary vascular endothelial cells, which triggers an inflammatory response in the donor heart [5]. In a mouse model of diabetes-induced cardiomyopathy, by examining the expression of key regulators of iron death, late glycosylation end-products, an important causative factor in diabetic cardiomyopathy, were found to induce iron death in engineered cardiac tissues, which was manifested by increased levels of PTGS2 and lipid peroxides and decreased levels of ferritin and SLC7A11 [6]. In an adriamycin-induced cardiomyopathy model, cardiac iron levels, lipid-derived ROS, and iron death biomarkers were significantly increased, whereas inhibition of iron death occurrence had a significant cardioprotective effect [7]. In addition, iron death plays an equally important role in the lipopolysaccharide-induced septic cardiomyopathy model, in which lipopolysaccharide triggers septic cardiomyopathy by inducing an increase in the expression of SFXN1 in myocardial mitochondrial membranes, which increases the production of mitochondrial ROS and leads to iron death [8]. In a mouse model of hypertrophic cardiomyopathy, genetic deletion of SLC7A11, a key regulator of iron death, exacerbates angiotensin-mediated cardiac fibrosis, hypertrophy, and dysfunction, which provides genetic evidence for the involvement of iron death in hypertrophic cardiomyopathy [9].
04 The role of iron death in inflammation
Unlike apoptosis, which is immunosilenced, iron death is immunogenic, and cells affected by iron death can release damage-associated molecular patterns and alarms that amplify cell death and promote a range of inflammation-related responses (Fig. 2). NAFLD is a severe fatty liver disease characterized by lobular inflammation, hepatocyte lipid accumulation, and balloon degeneration, and in a mouse model, protein levels of pro-inflammatory cytokines (including TNFα, IL-1β, and IL-6) were found to be significantly elevated in liver tissues by inducing iron death, which was dramatically ameliorated by an iron death inhibitor that reduced NAFLD severity[10] . In inflammatory dermatoses, treatment of inflammatory dermatoses with iron death inhibitors resulted in significant improvement of the skin and down-regulation of GPX4 expression, up-regulation of Nrf2 downstream targets, increased intracellular iron input, and down-regulation of expression of the inflammatory factor IL-22[11]. In addition, in the oxalic acid-induced acute kidney injury (AKI) model in mice, an iron death inhibitor suppressed neutrophil infiltration and the expression of pro-inflammatory cytokines CXCL-2 and IL-6 [12]. Recently, it has been found that secondary brain damage caused after cerebral hemorrhage is related to iron death, and iron death in the brain can cause inflammatory responses in a rat model of cerebral hemorrhage, while iron death inhibitor treatment can significantly reduce the levels of ROS and inflammatory factors such as IL-1β and TNFα, reduce inflammation and improve neuronal function in rats with cerebral hemorrhage injury [13]. In a mouse model of radiation-induced pulmonary fibrosis, iron death inhibitor significantly reduced the expression of TNFα, IL-6, IL-10, and TGF-β1 and blocked radiation-induced pulmonary fibrosis by activating the Nrf2/HO-1 signaling axis [14].
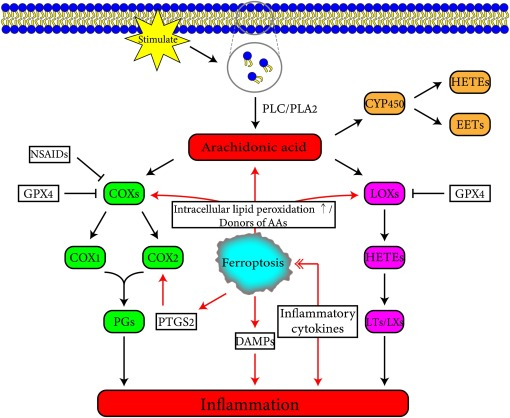
▲Fig. 2 Interaction between iron death and inflammation [15]
05 Role of Iron Death in Cancer
In the last decade, many studies have shown that iron death is associated with the development of various types of tumors and the response to treatment, and can trigger inflammation-associated immunosuppression in the tumor microenvironment, thereby favoring tumor growth [16]. In tumor tissues of patients with plasmacytoid ovarian cancer, iron transport protein (FPN) was found to be decreased and transferrin receptor (TFR1) was increased, and treatment with iron death inducers inhibited tumor growth and intraperitoneal dissemination of tumor cells in vivo [17]. Another study reported that cilazin and lapatinib synergistically induced breast cancer cell death mediated by iron death by increasing iron-dependent ROS production [18]. Cisplatin treatment induces iron death by reducing glutathione depletion and glutathione peroxidase inactivation in lung cancer cells, in which the combination of cisplatin and iron death inducers significantly increased antitumor activity [19]. In addition, it has been reported that low doses of iron death inducers can significantly increase the ability of adriamycin and cytarabine to kill acute myeloid leukemia cells by stimulating necrotic apoptosis and iron death [20].
06 Mesenchymal stem cells mitigate disease progression by inhibiting iron death
Mesenchymal stem cells (MSCs) have a high self-renewal capacity, multilineage differentiation potential, and immunomodulatory properties, and these unique features make them a powerful tool for research and clinical applications. In acute liver injury, treatment with MSCs and derived exosomes (MSC-Exo) achieved pathological remission and inhibited lipid peroxidation production, downregulated peroxisomal synthase 2 and lipoxygenase mRNA levels in primary hepatocytes and mouse livers, restored SLC7A11 protein levels, and prevented CCl4-induced iron death in hepatocytes [21]. In a mouse model of acute myocardial infarction, iron death occurs in cardiomyocytes after ischemic and hypoxic injury, whereas human umbilical cord blood-derived MSC exosomes can attenuate myocardial injury by inhibiting iron death in acute myocardial infarction mice [22]. Iron death also plays an important role in the development of hepatic fibrosis, and hepatic stellate cell activation can cause hepatic fibrosis, and human umbilical cord MSCs and their secreted exosomes can reduce hepatic stellate cell activation and attenuate hepatic fibrosis in animal models by promoting iron death triggered by ROS formation in human hepatic stellate cells, mitochondrial dysfunction, Fe2+ release, and lipid peroxidation [23].
▲Bone Marrow Mesenchymal Stem Cells (copyrighted image, please do not reproduce)
It has been shown that iron death is associated with the pathophysiology of traumatic brain injury and that the levels of biomarkers related to iron death in tissues of repetitive mild traumatic brain injury showed time-dependent alterations, such as abnormal iron metabolism, glutathione peroxidase (GPX) inactivation, decreased GPX4 levels, and increased lipid peroxidation, whereas MSC treatment significantly reduced the above mentioned repetitive mild traumatic brain injury mediated alterations, neuronal damage, pathologic protein deposition, and improved cognitive function [24]. Acute liver injury is characterized by severe metabolic dysfunction caused by extensive hepatocyte damage, and in vivo and in vitro experiments have demonstrated that MSC exosome treatment inhibits iron death induced by reactive oxygen species and lipid peroxidation in liver tissues, and significantly attenuates D-galactosamine- and lipopolysaccharide-induced liver injury [25]. In the porcine cardiac arrest model, the expression level of ACSL4 and iron deposition level of the heart of all resuscitated animals were significantly increased, and the expression level of GPX4 was significantly decreased, which triggered iron death, and the treatment of MSCs significantly reduced the iron death after resuscitation from cardiac arrest, and significantly alleviated the post-resuscitation cardiac injury in pigs [26]. Neutrophil extracellular traps (NETs) play an important role in abdominal aortic aneurysm formation, and neutrophil extracellular traps promote abdominal aortic aneurysm formation by inducing iron death in smooth muscle cells through inhibition of the PI3K/AKT pathway. In a mouse model, MSC exosomes alleviate iron death by decreasing the release of neutrophil extracellular traps through their immune regulation and regenerative ability, attenuating abdominal aortic aneurysm formation [27].
07 Summarize
Iron death is a novel non-apoptotic form of regulated cell death characterized by a massive accumulation of lipid peroxides that triggers a variety of diseases. In the future, iron death may be found in more and more diseases and play an important role in the pathophysiological process, and studying the therapeutic targets of diseases from the perspective of iron death can provide new clinical solutions for the treatment of diseases. At present, there have been many clinical studies on MSC therapy in China, and the studies confirmed that MSCs also play an important role in iron death-induced disease damage, and it is believed that in the near future, stem cell therapies will play a greater role in the treatment of diseases in a surprising way.


