25
2023
-
08
Principles of Transdermal Cell Therapy - TCR-T
Recently, Cell Reports Medicine, an internationally renowned journal, released the Phase I clinical research results of TAEST16001, the first TCR-T therapy product in China [1].TAEST16001 is used for the treatment of advanced soft-tissue sarcoma, which is a key step towards the clinical translation of ACT in the field of solid tumours, and once again ignited people's confidence in the treatment of solid tumours with TCR-T therapies. confidence in TCR-T therapy for solid tumours. In this issue, let's talk about the ins and outs of TCR-T therapy.

01 Preface
Recently, Cell Reports Medicine, an internationally renowned journal, released the Phase I clinical research results of TAEST16001, the first TCR-T therapy product in China [1].TAEST16001 is used for the treatment of advanced soft-tissue sarcoma, which is a key step towards the clinical translation of ACT in the field of solid tumours, and once again ignited people's confidence in the treatment of solid tumours with TCR-T therapies. confidence in TCR-T therapy for solid tumours. In this issue, let's talk about the ins and outs of TCR-T therapy.
The tumour microenvironment (TME) of solid tumours is rich in microscopic stroma and immunosuppressive cells, which can protect the tumour tissue against the attack of immune cells. In the microenvironment of pancreatic cancer, for example, increased levels of hyaluronic acid (HA) impede the infiltration of immune cells. Immune checkpoint receptors on tumour cells or immunosuppressive cells can inhibit T cells by binding to negative regulatory ligands on T cells. And in this tumour microenvironment, which lacks essential amino acids and low oxygen, T cells become senescent and depleted. Therefore, there is no cure for solid tumours, and their progression can only be controlled by various means. Although chimeric antigen receptor T-cell (CAR-T) therapy has made great breakthroughs in haematological tumours, there is still no greater breakthrough in the treatment of solid tumours. As a result, researchers have turned their hopes for a solid tumour cure to TCR-T, an alternative approach to over-the-counter cell therapy (ACT).
02 What is TCR-T therapy
T-cell receptor chimeric T-cell (TCR-T) therapy is based on the same idea as CAR-T, where engineered T-cells capable of specifically recognising antigens are obtained by modifying the T-cells in vitro, and the final expressed T-cells are able to target and clear the tumour cells for the treatment of cancer [2] (Figure 1).
Therapeutic ideas:
(1) Sufficient blood is drawn from the patient to obtain peripheral mononuclear cells (PBMC);
(2) Isolation & purification of T cells from PBMC;
(3) In vitro culture to expand T cells;
(4) Introduction of TCR or CAR gene fragments to be modified into T cells (via viral vectors such as lentivirus and retrovirus);
(5) After continued expansion and quality control, the modified TCR-T/CAR-T is infused back into the patient.
Unlike CAR-T therapies, which recognise tumour cell surface antigenic targets via the CAR structure, TCR-T therapies rely on the binding of T-cell receptors (TCRs) to intracellular antigens presented by MHCs to recognise tumour cells. To understand the mechanism of TCR-T, it is important to understand the structure and working mechanism of the major histocompatibility complex (MHC) and the T-cell receptor (TCR).
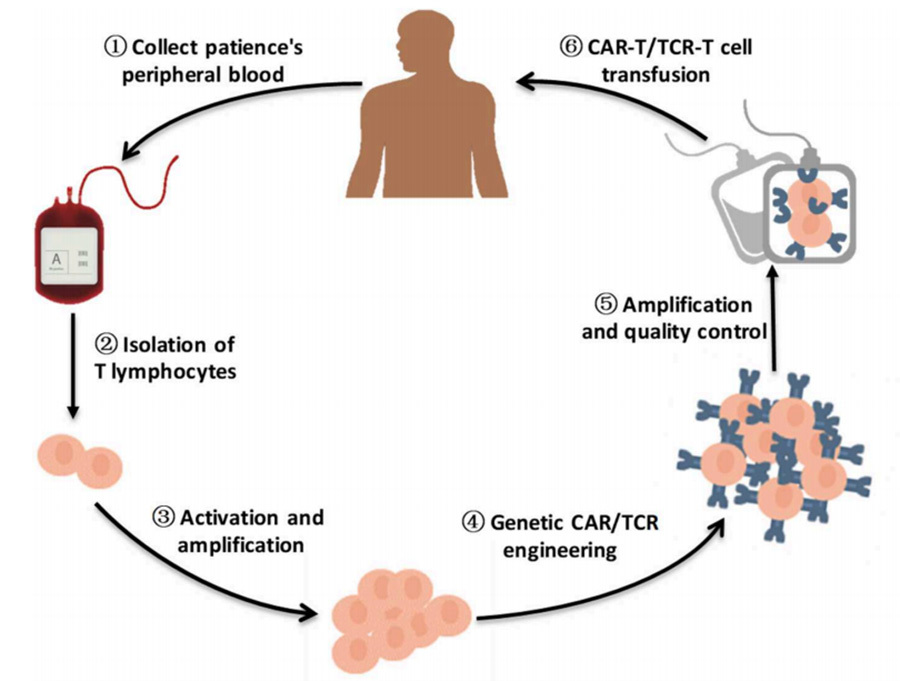
▲Figure 1. Brief flowchart of engineered T-cell therapy
03 Structure and function of MHC
There are two types of MHC, MHC-I and MHC-II, encoded by the human leukocyte antigen (HLA) locus on the short arm of human chromosome 6, which are expressed to varying degrees on the cell surface. Among them, HLA-A, HLA-B and HLA-C encode MHC-I type I molecules, and HLA-DR, HLA-DQ and HLA-DP encode MHC-II type II molecules [3] (Fig. 2).The HLA genes play a very important role in the immune system, and the human immune system distinguishes between self and non-self cells by the products encoded by the HLA genes. Mismatches between the HLA genes of an organ donor and the HLA genes between patients usually result in immune rejection.
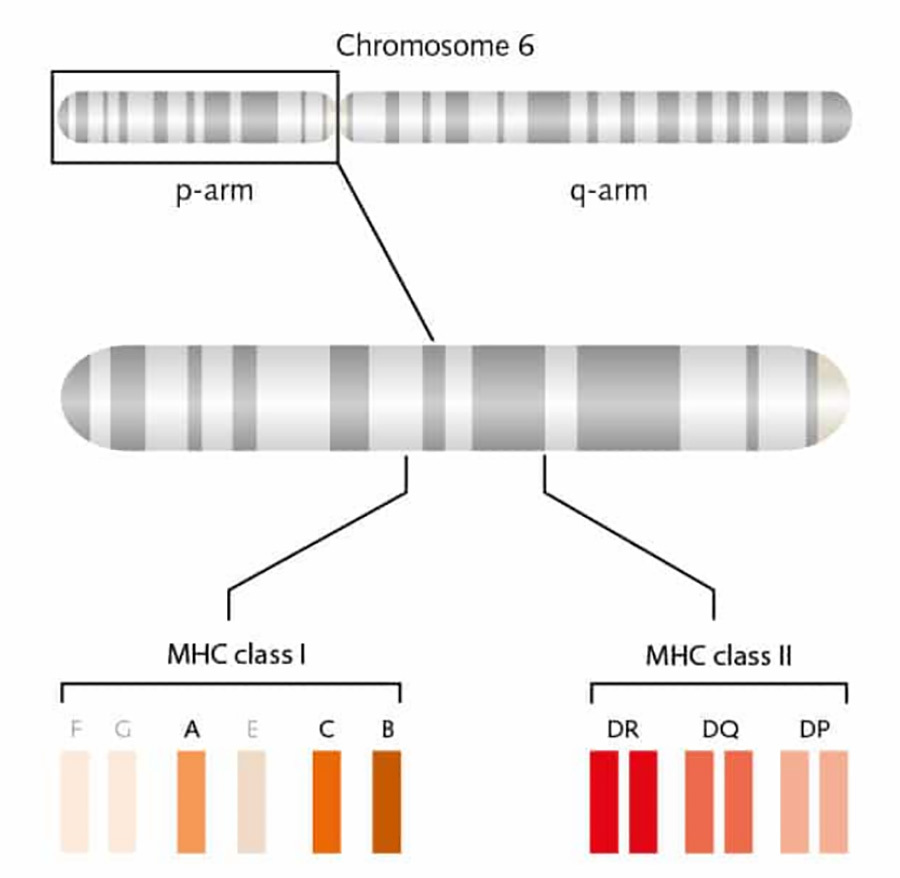
▲Figure 2. Schematic representation of the human HLA locus
MHC-I is responsible for the presentation of endogenous antigen signalling molecules, it is expressed on the surface of almost all nucleated cells and consists of an α-chain and β-globin, where the α1 and α2 domains form a groove, which is the peptide binding cleft, which permits antigenic polypeptide fragments to bind to and be presented on the surface of the cell membrane.MHC-II is responsible for the presentation of exogenous signalling molecules, and is expressed on the surface of antigen-presenting cells (APC), such as monocyte-macrophages, dendritic cells and B cells. MHC-II is responsible for the presentation of exogenous signalling molecules and is expressed on the surface of antigen-presenting cells (APCs), such as monocyte-macrophages, dendritic cells, and B-cells.MHC-II consists of two chains, α and β, where the groove formed between α1 and β1 is involved in the binding of antigenic polypeptide fragments [4] (Figure III).
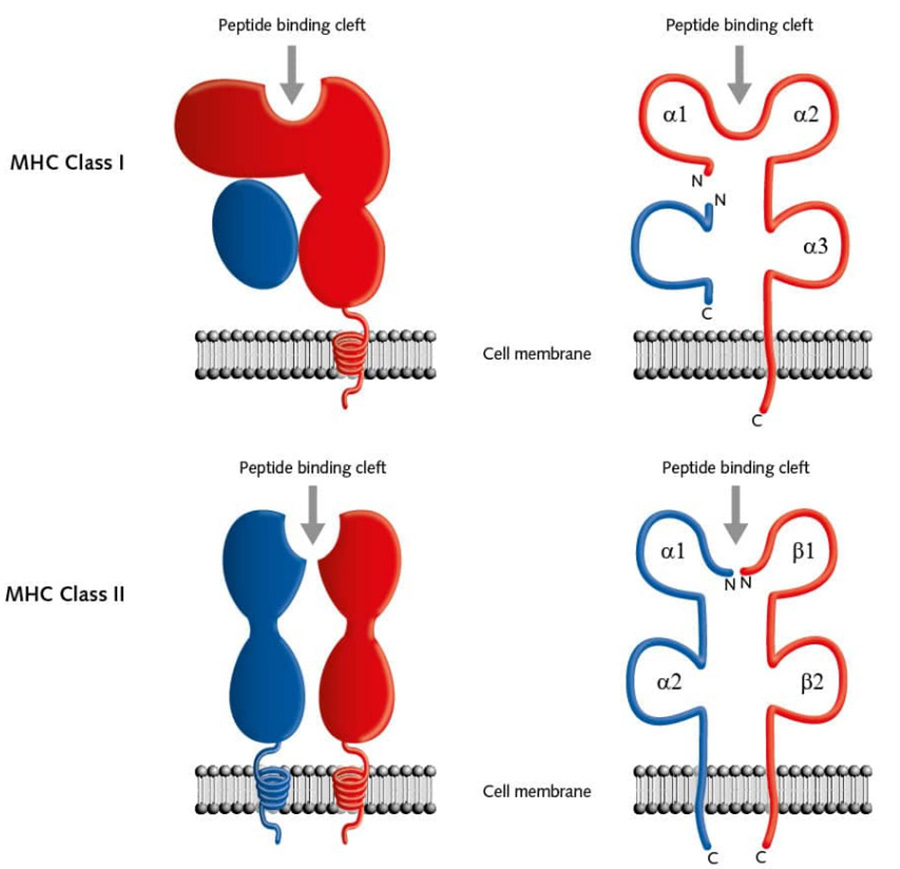
▲Figure 3. Schematic structure of MHC (HLA)
Endogenous antigens are proteins produced by the cell itself, such as protein shells synthesised using host cells after viral infection or tumour proteins from cancerous lesions. Within the cell, these endogenous proteins form small molecular peptides when processed by their own cells, which then bind to MHC-I to form the pMHC-I complex and are presented to the surface of the cell membrane to bind to CD8+ T-cells, which mediate apoptosis or programmed cell death (Fig. 4, left).
When antigens are not synthesised endogenously by the cell but are invaded from the outside such as by bacterial or fungal infections, APC cells take them into the cell by phagocytosis and digest them into small fragments of peptide chain antigenic molecules. The resulting antigenic molecules bind to MHC-II to form the pMHC-II complex and are presented to the surface of the cell membrane, where the pMHC-II complex will bind to CD4+ T cells to generate a number of columns of immune responses to clear the antigen (Fig. 4 right).
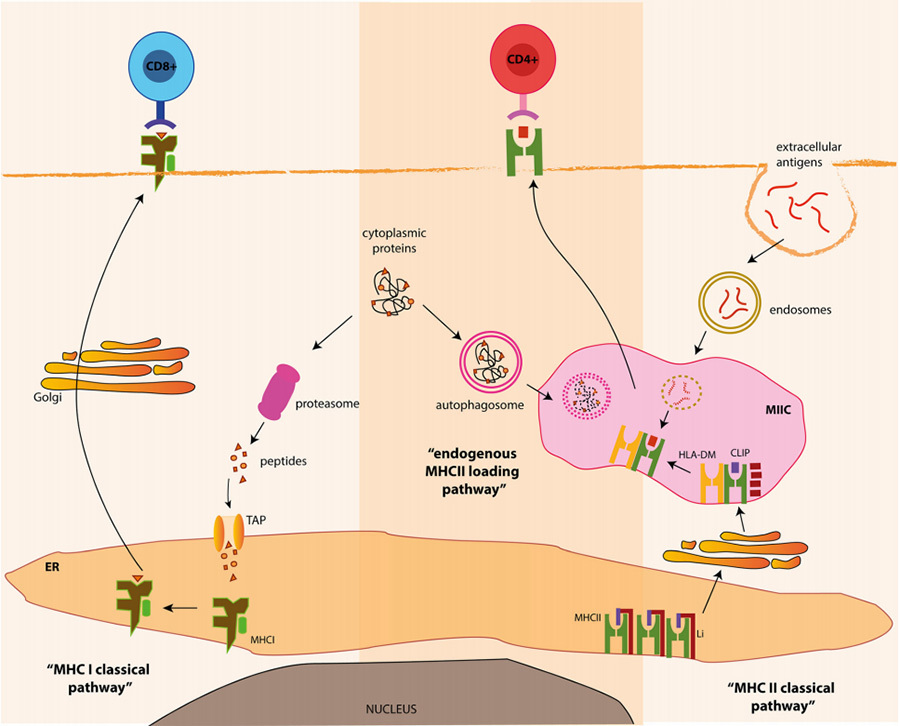
▲Figure 4 Classical pathway diagram of MHC [5]
04Structure and function of TCR
Through the above introduction, we understand that TCR-T recognition of antigens depends on MHC to present intracellular antigen molecules to the surface of the cell membrane, so MHC is an essential component for the function of TCR-T. TCR complex, is another important component of TCR-T. In order to activate T lymphocytes, the TCR complex must interact with the antigen-carrying MHC complex (pMHC).
The TCR used to genetically engineer the TCR-T consists of heterodimers of α and β chains, which are necessary structures for the TCR to bind to pMHC, but this is not sufficient to cause activation of T cells.T cell activation is dependent on signalling by the CD3 molecule, which consists of two heterodimers, CD3ε and CD3δ, CD3ε and CD3γ, and one homodimer, CD3ζ (CD3 activation of T cells is dependent on the phosphorylation of the immunoreceptor complex amino acid activation motif (ITAM) of each subunit (Figure 5).
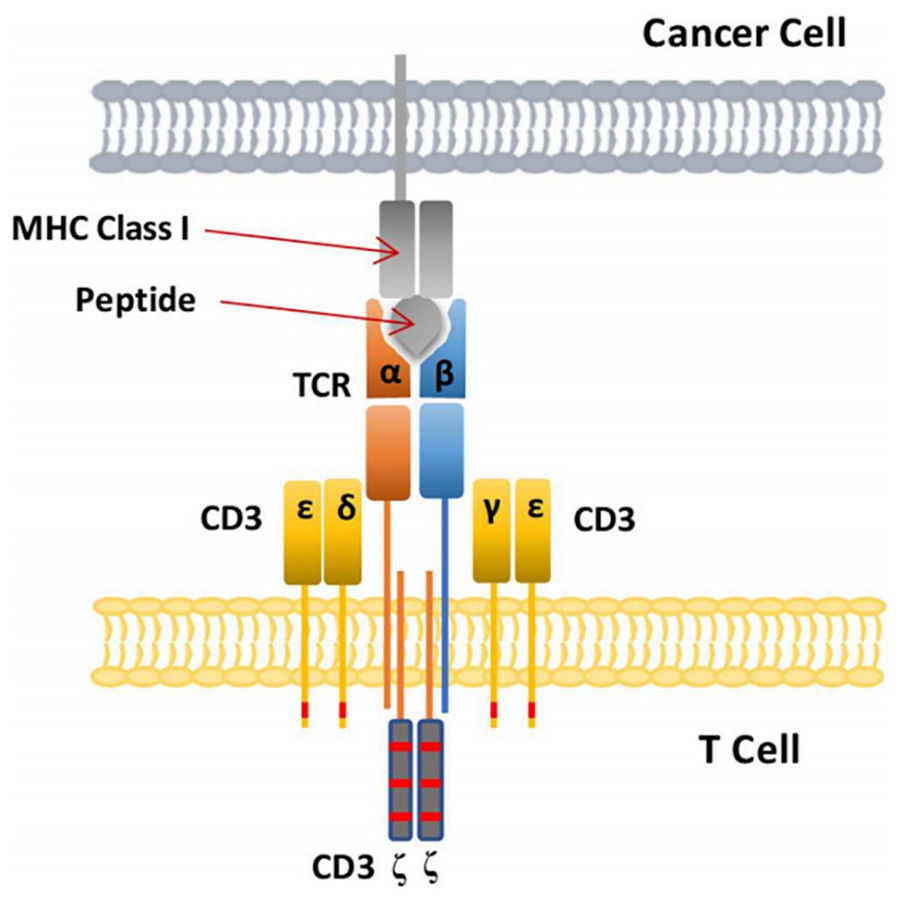
▲Figure V. Schematic representation of TCR binding to pMHC in tumour cells [2]
05 TCRs and CARs
TCR-T and CAR-T are both genetically engineered T-cells with the ability to specifically identify and remove tumours, and then infused back into the body for the purpose of treating cancer.The target antigens recognised by CAR-T are cell-surface proteins, and the number of these target proteins is limited.TCR-T can recognise intracellular polypeptide fragments delivered by MHC molecules, and therefore the range of target antigens for TCR-T is much wider. The core of TCR-T technology is to modify the binding of TCR to tumour antigens, for which the natural TCR in the human body has a relatively low affinity and therefore cannot recognise and kill tumour cells. Artificially designed high-affinity TCRs enhance specific recognition and affinity in recognising tumour cells.
However, TCR-T therapy is MHC cell-limited and relies on the presentation of MHC molecules to recognise and activate T cells, which is a disadvantage of TCR-T. MHC is encoded by the HLA gene, which is the most polymorphic gene in the human genome, and it has been estimated that more than 20,000 HLA I human alleles have been identified to date. Patients undergoing TCR-T therapy must not only express the targeted antigen, but also the corresponding HLA allele antigen. As a result, TCR-T therapy typically uses TCRs that are limited to relatively common HLA alleles.
06 Outlook
On 25 January 2022, the world's first TCR-T therapy product was approved for marketing by the FAD, which is Immunocore's Kimmtrak (tebentafusp-tebn, IMCgp100) for adult patients with unresectable or metastatic uveal melanoma (mUM) that is HLA-A*02:01 positive. This is a huge breakthrough for over-the-counter cell therapy in solid tumours. Domestic companies such as WuXi Juno, Fosun Kite, and Xiangxue Life Sciences have also made relevant layouts in TCR-T therapy.TAEST16001 is a product developed by Xiangxue Life Sciences, which is currently undergoing clinical phase II trials.TCR-T still faces many challenges in oncology treatment, such as the need to identify more tumour-cell specific antigens and recognised TCRs, and how to improve its relationship with antigens by modification of TCR to improve its specific recognition and affinity for antigens and other issues still need further research. In any case, overcell therapy represented by TCR-T has demonstrated its great advantages in tumour therapy and is a key technology for solving cancer.


