16
2023
-
08
Principles of Cellular Immunotherapy - CAR-T
In the past decades, scientific research on the anti-tumour mechanism of immune cells has made rapid development, and Adoptive Cell Therapy (ACT) has become the most promising method to cure cancer.ACT therapy refers to the method of collecting the patient's blood, isolating the immune cells in vitro, and infusing them back into the patient's body through modification, proliferation, cultivation, and identification to achieve the purpose of treating cancer. ACT therapy mainly includes TILs, CAR-T, TCR-T, etc. CAR-T (Chimeric Antibody Therapy) is the most promising method to treat cancer.

01 Introduction
In the past decades, scientific research on the anti-tumour mechanism of immune cells has made rapid development, and Adoptive Cell Therapy (ACT) has become the most promising method to cure cancer.ACT therapy refers to the method of collecting the patient's blood, isolating the immune cells in vitro, and infusing them back into the patient's body through modification, proliferation, cultivation, and identification to achieve the purpose of treating cancer. ACT therapy mainly includes TILs, CAR-T, TCR-T, etc. CAR-T (Chimeric Antibody Therapy) is the most promising method to treat cancer.
CAR-T (Chimeric Antigen Receptor T-cell, CAR-T) therapy, i.e. Chimeric Antigen Receptor T-cell therapy, is one of the newest and most promising methods for cancer treatment at present, which can enhance the body's immune system to fight against cancer.CAR-T therapy, by means of genetic engineering, chimerises a certain specific tumour antigen receptor gene on the cell membrane of the T-cells to form a modified tumour antigen receptor gene. antigen receptor gene on the cell membrane of T cells to form modified T cells, which in turn can specifically recognise and bind antigens on the surface of tumour cells to achieve specific killing of tumour cells (Figure 1). Unlike T cells, the CAR structure of CAR-T cells recognises antigens on the surface of tumour cells and directs T cells to perform cytotoxic functions without relying on MHC molecules (major histocompatibility complex), thus CAT-T cells have a wider range of targets than natural T cells.
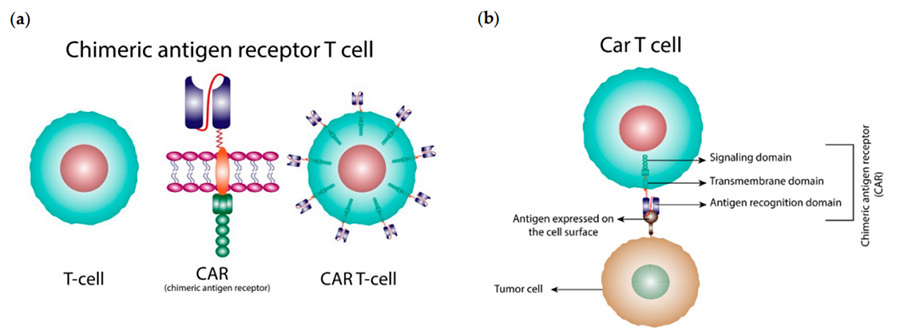
▲Figure 1: Schematic diagram of the CAT-T [1].
CAR is an antigen-targeting protein receptor that can fuse with T cells and specifically recognise antigens on tumour cells.The design of CAR structure is the key to CAR-T therapy. A typical CAR structure consists of three main parts: the extracellular domain, the transmembrane domain and the intracellular domain (Figure II).
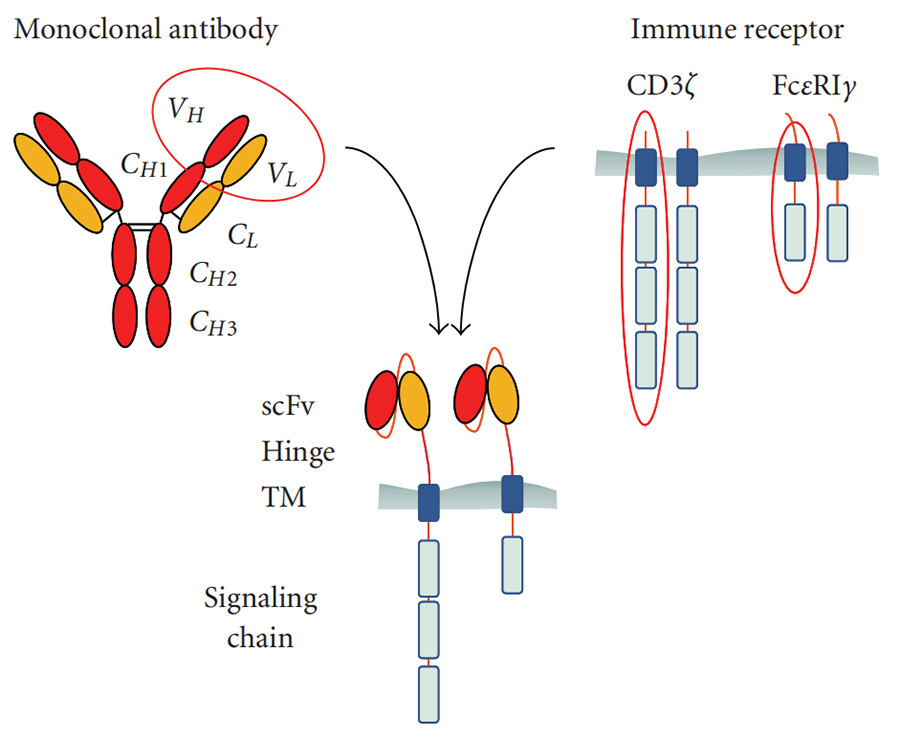
▲Figure 2: Structure of the chimeric antigen receptor (CAR) [2]
The extracellular domains are Single-Chain Variable Fragment ,scFv, and Hinge domain of monoclonal antibody. scFv is responsible for recognising and binding to Tumor-Associated Antigen (TAA) on the surface of the tumour cells. hinge is responsible for connecting scFv to the transmembrane Hinge is responsible for connecting scFv to the transmembrane structural domain (TM).
The transmembrane domain is responsible for anchoring the CAR structure to the T cell membrane and has a stabilising effect on the CAR structure, and there is evidence that it also enhances T cell activation [3].
The intracellular domain consists of two parts, the Signaling Activating Structural Domain (SASD) and one or more Co-Stimulatory Structural Domains (CSDs). The Signaling Domain is responsible for the activation of T cells, and this structure is usually CD3ζ. The process of T cell activation is dependent on the phosphorylation of the immunoreceptor complex amino acid activation motif (ITAM) in CD3ζ. In addition to CD3ζ, some studies have used the Fc receptor of the immunoglobulin (Ig) E-γ structural domain as a signal-activating structural domain for CAR, but have found this structure to be less effective. However, relying on CD3ζ in the signal-activating structural domain alone is not sufficient to activate T cells to a certain level and sustain CAR-T cell proliferation. The co-stimulatory structural domain (which is lacking in first-generation CAR-T) usually originates from the signaling subunits of T cells, such as from the CD28 receptor family (CD28, ICOS) or the tumor necrosis factor receptor family (4-1BB, OX40, CD27), and its function is to conduct extracellular binding signals to the T cells, initiate downstream signaling cascade reactions, provide a second signal for T cell activation which enables T cells to proliferate continuously and release cytokines to improve the anti-tumour ability of T cells.
In short, each of the three parts of the CAR structure performs its own function. scFv acts as an antigen recogniser, which can recognise and bind to the target cells; the transmembrane structural domain is equivalent to the "scaffold" of the CAR, which firmly fixes the CAR structure to the T cells; the signal-activating structural domain and the co-stimulatory structural domain work together to activate the T cells, proliferate and guide their killing. The signal activation domain and the co-stimulatory domain together activate T cells, proliferate and guide them to kill tumour cells, and at the same time induce endogenous immune cells to clear tumour cells. Upon antigen recognition and binding, a stimulatory signal is generated and transmitted to the intracellular signalling domain, and T cells are activated and function.
03 Evolution of different generations of CAR-T
After nearly three decades of development and continuous technological innovation, CAR is now in its fifth generation, which aims to improve the safety of therapy by reducing toxicity and non-specific antigen recognition, increase efficiency by stimulating proliferation, activation, and generation of memory phenotypes in CAR-T cells, and provide immunomodulation for optimal CAR-T cell action (Figure III).
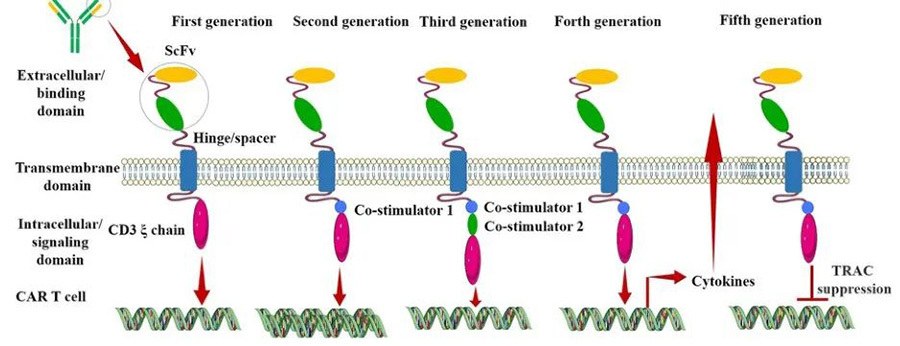
▲Figure 3; Evolution of CAR structure over generations [4]
First Generation CAR
The first-generation CAR structure has extracellular scFv as an antigen recognition binding structural domain and intracellular CD3ζ as an intracellular signalling activation structural domain. Although first-generation CAR-T could initiate cytotoxic antitumour responses in transplanted T cells, the CAR structure could not produce sufficient levels of interleukin (IL)-2 due to the lack of a co-stimulatory structural domain, resulting in low levels of cytotoxicity and proliferation of T cells.
Second-generation CARs
Based on the CD3ζ signalling domain, the second generation of CARs added a co-stimulatory signalling structural domain capable of activating T-cells, which greatly improved T-cell proliferation and survival time. For example, CD28 can deliver a very strong activation signal, which can enable T cells to reach a high level of killing activity in a relatively short period of time; 4-1BB delivers a more persistent activation signal, which can enable sustained killing of tumour cells by T cells. However, due to the fact that second-generation CAR-T cells use retroviruses as infectious vectors, the length of the transgene fragments they can carry is limited, and they cannot simultaneously transfer both CD28 and 4-1BB into T lymphocytes at the same time, so they can only choose between the two.
Third-generation CAR
The third generation of CAR-T uses lentiviruses with larger DNA loads as infectious vectors, e.g., DNA fragments of both CD28 and 4-1BB can be introduced into T cells at the same time, so the third generation of CAR structure contains two co-stimulatory structural domains, which theoretically solves the problem of higher activation intensity of CAR-T more durable survivability. However, there is still no good solution to the safety issues caused by the high level of persistence of CAR-T that may lead to its attack on the autoimmune system.
Fourth-generation CARs
The fourth generation of CAR is designed from the perspective of precision treatment of oncological diseases. For example, solid tumors in the process of chronic development will create a microenvironment (TME), resulting in CAR-T can not enter the tumor, so CAR-T therapy in the treatment of solid tumors has not been very effective. TRUCK CAR-T will cytokines (IL-12) or chemokines into the CAR, can increase the infiltration of T-cells in the tumor tissues, and at the same time, to recruit the body's other immune cells to clear the tumor cells; in some cases, the CAR will be used for the treatment of tumor cells; in some cases, the CAR may attack the body's own immune system, which may lead to safety problems. Tumour cells are removed; in some studies, a suicide gene or a gene sensitive to certain drugs is attached to the CAR structure to ensure that the CAR-T can be removed from the body after treatment without accidentally injuring normal cells in the body, improving the safety and controllability of CAR-T therapy.
Fifth Generation CAR
The fifth generation CAR-T is a general-purpose CAR-T, which avoids the occurrence of graft-versus-host response (GVHD) by knocking out the TRAC gene, which leads to the removal of the T-cell receptor α (TCR-α) and β (TCR-β) chains, implying that T-cell receptors (TCRs) on the surface of T-cells are removed.
04 Summary
Since the FDA approved the CD19 CAR-T product Novartis (Kymriah) on the market in 2017, CAR-T cell therapy has entered a phase of rapid development, but the products currently approved on the market are all second-generation CAR-T. There is still a long way to go for CAR-T to become popular in the market.
Safety is the first issue to be addressed by CAR-T, such as off-target effect, cytokine release syndrome (CRS) and neurotoxicity (NTX); currently marketed CAR-T products are focused on the treatment of haematological tumours, while the treatment of solid tumours has not yet achieved a major breakthrough. 2021 China's NMPA approved Fosun Kate's Argirenzai injection and WuXi Juno's Ruigi Orenzai injection. Both CAR-T products target CD19. Although CAR-T targeting CD19 is very effective, it is only limited to the treatment of B-cell related haematological tumours, and more specific targets need to be developed to address the treatment of solid tumours; expensive price is also a huge challenge for CAR-T, such as 1.2 million RMB per injection for Alkirenzai injection and 1.2 million RMB per injection for Rigiorenzai. The expensive price is also a great challenge for CAR-T, such as 1.2 million RMB for Argirenzide injection and 1.29 million RMB for Rigiorenzide injection, which makes most of the cancer patients reluctant to take it.


